Our Story
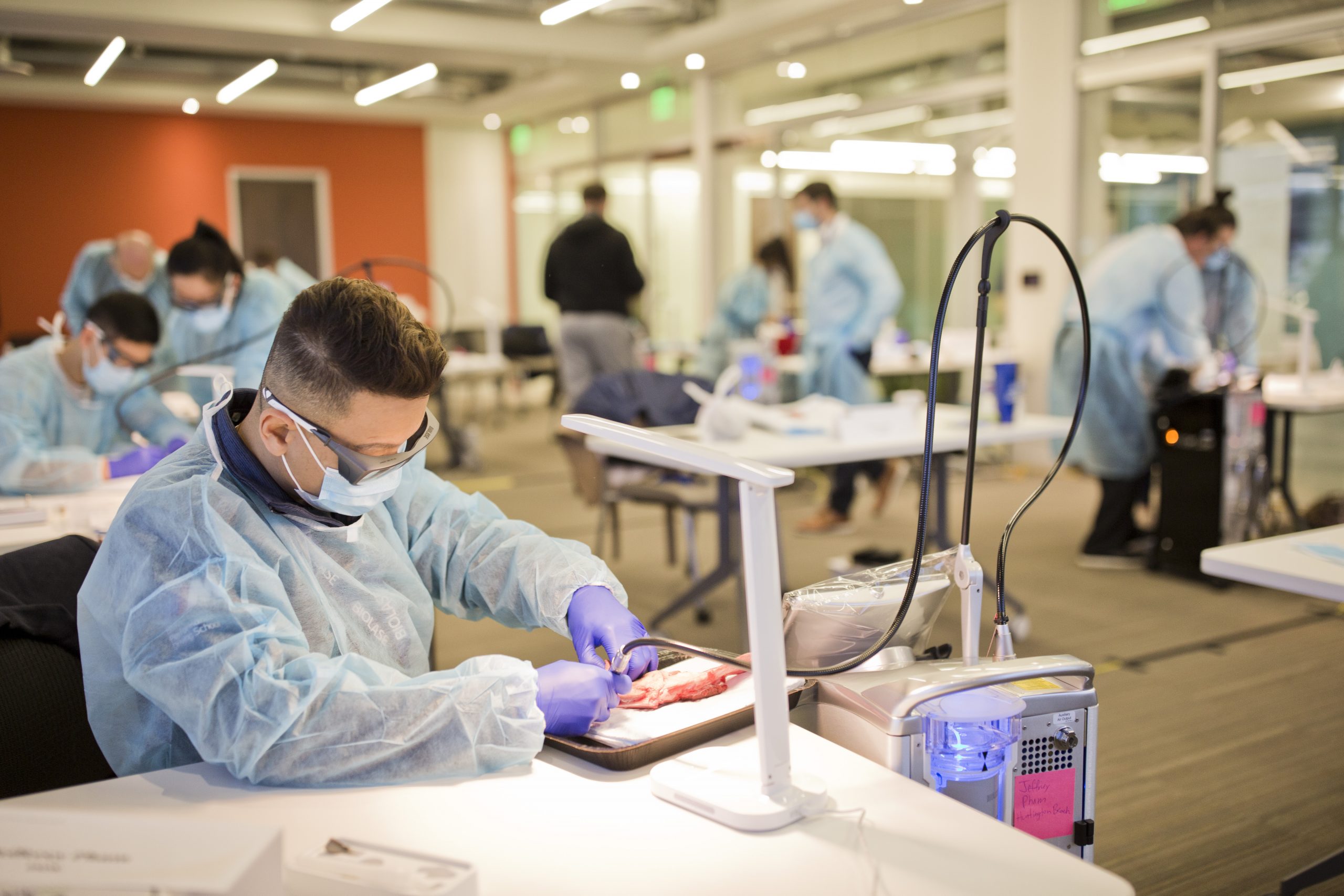
BIOLASE is a medical device company and the global market leader in the manufacturing and marketing of proprietary dental laser systems. Since our founding, we’ve sold over 43,300 laser systems in more than 80 countries, transforming dental care through innovative technology that delivers clinically superior, patient-friendly results compared to traditional methods.
For decades, BIOLASE has set the standard for dental laser excellence. As a proud member of the MegaGen family, we are now poised to redefine the future of dentistry once again. Under the leadership of Dr. Kwang Bum Park, CEO of MegaGen & BIOLASE, this new chapter brings a renewed commitment to the quality, innovation, and customer care that defined the golden age of BIOLASE.
Together with MegaGen, we are uniting world-class laser and implant technologies to elevate clinical outcomes and empower dental professionals worldwide.